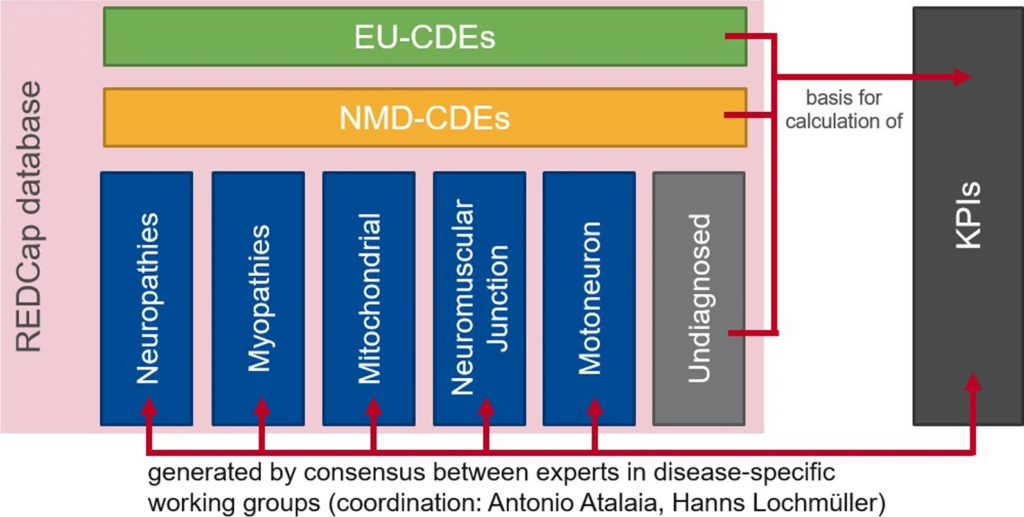
Sets of data items in the ERN EURO-NMD Registry.
The registry has three data levels:
- EU-CDEs: these are the set of Common Data Elements that were chosen by the Joint Research Centre and the European Platform on Rare Disease Registration for collection by all the European Reference Networks. These CDEs need to be collected once per year for each patient seen at the EURO-NMD’s healthcare providers.
- NMD-CDEs: this dataset contains all the cross-neuromuscular data elements that are useful for any neuromuscular condition. This dataset is recommended but not mandatory until 2026.
- DS-DEs: disease specific data elements are fields specific for each condition or group of conditions, as defined by the 5 working groups.