An overview of the technical development of the EURO-NMD Registry Hub – user-friendly, content-rich and FAIR. A review of the Work Package 4 progress by Dagmar Jäger, Hanns Lochmüller and Adrian Tassoni
As part of the EURO-NMD Registry Hub project we are currently developing a new, centralised patient registry for all neuromuscular disorders that will be used by health care providers (HCP) across Europe. The primary objective of the EURO-NMD registry is to improve care and outcomes for all patients with neuromuscular disorders. Once operational, this registry will be part of a larger registry landscape of neuromuscular registries, which will all be connected through a single registry hub. In order to make the data collected in the EURO-NMD registry ready for sharing applications we follow the FAIR data principles from the start [1].
We have divided our work into four blocks:
- The definition of user requirements including the general and disease specific data elements that need to be collected in close collaboration with the 5 EURO-NMD working groups on myopathies, neuropathies, neuromuscular junction disorders, motorneuron diseases and mitochondrial diseases,
- the FAIRification process of these data elements in close collaboration with the WP4 team at the Radboud university medical center in Nijmegen, the FAIR data stewards and the European Joint Programme on Rare Diseases (EJP RD),
- the implementation of the actual registry software together with the tools to make the registry FAIR and
- a test and refinement phase with EURO-NMD clinicians and members.
At the end of the project period (April 2023), we will have a ready to use system in place to be rolled out to HCPs across Europe.
Data elements
We created a codebook, a structured document, to collect and harmonise all data elements that we received from the working groups for the five different disease groups that will be covered by the registry (neuropathies, myopathies, mitochondrial diseases, neuromuscular junction disorders, motoneuron diseases). We also added the mandatory common data elements (CDE) [2] specified by the European Platform for Rare Disease Registration (EU RD Platform). The codebook also contains a section for undiagnosed cases.
FAIRification
In order to implement the FAIR principles, we started by adding ontology codes to each element in the codebook. First, we focused on the CDEs. These CDEs are the minimum amount of information that should be provided in a FAIR way. We are very grateful that the data stewards of the EJP RD have already done good groundwork, especially with regard to the FAIRification of CDEs and the tools they have developed to facilitate the implementation of the FAIR principles. We have particularly benefited from the work of the VASCA group with their codebook [3], which we have used as a template for our FAIR-enabled codebook and the CDE-in-a-Box tool [4] developed centrally by the EJP RD team.
For an in depth look at the FAIR data principles and the CDE-in-a-box tool click here
Tools
We decided on REDCap [6] as the platform for data entry and started implementing all the data elements in it. We have chosen a multi-arm project structure to differentiate between disease groups. This allowed us to display only the data elements relevant to a given patient.
The longitudinal design we have chosen allows HCPs to enter further information or updates on previously entered data at flexibly selectable times. We recommend annual updates for the majority of data elements. REDCap also includes the possibility for patients to participate in data entry themselves. The respective centre can generate a survey link for a form and send it to the patient. The patient can then open and edit the form in a browser via this link.
To make the entered data FAIR, we set up the CDE-in-a-Box tool. This tool enables the automatic conversion of table-based data into machine-readable “semantic” data and already contains ready-to-use procedures for mapping the CDEs. All we had to do to accomplish this was to implement a mapping from our own REDCap structure to the structure requested by the cde-in-a-box tool. CDE-in-a-Box also automatically updates the transformed data into a graph database, which forms the gateway from the registry to the central registry hub.
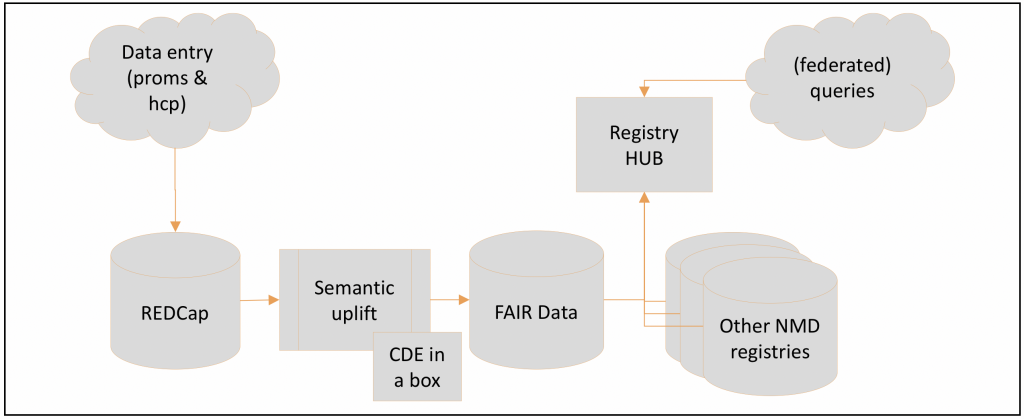
With all the components of this FAIRification pipeline in place, the system can automatically provide a FAIR data output directly after data entry in REDCap. Thus, the registry can be used in combined data analysis queries with other registries within the EURO-NMD registry hub and with other ERN registries applying the same FAIR structure. This is complemented by an access policy and consideration of data protection, legal and ethical principles. We hope that through this pooling of data we can achieve larger case numbers to provide a good basis for new insights into neuromuscular diseases and for improvement of patient care.
Testing and Refinement
Our FAIRification process is still under development but we have already transitioned the system into the testing phase. We expect to receive additional comments about the content and functionality in the near future as now additional testers, especially health care providers are starting to evaluate the system. We are setting up forms and questionnaires to receive structured feedback from the testers. This feedback will then be collected, reviewed and implemented. If you are connected to our project in any way and are interested in testing our system, please feel free to contact us.
By the end of the project, we hope to have developed a useful system that reflects the needs of the ERN and that it provides a better insight into the European neuromuscular healthcare landscape.
References
- Wilkinson, M., Dumontier, M., Aalbersberg, I. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3, 160018 (2016). https://doi.org/10.1038/sdata.2016.18
- EU RD Platform. (n.d.). Set of Common Data Elements. Retrieved December 9, 2021, from https://eu-rd-platform.jrc.ec.europa.eu/set-of-common-data-elements_en
- ERN VASCERN. (2020, June 25). EJP RD tool for fairification of patient registries, made for the Vasca Registry, now available! Retrieved December 9, 2021, from https://vascern.eu/ejp-rd-tool-for-fairification-of-patient-registries-made-for-the-vasca-registry-now-available-for-use/.
- “CDE in a box” is a collection of software applications which enables creation, storing and publishing of “Common Data Elements” according to the CDE semantic model https://github.com/markwilkinson/temp-cde-in-a-box
- van Lin, N. (2021, November 26). Innovative and Sustainable FAIR solution for ERN Euro-NMD. Retrieved December 9, 2021, from https://registry.ern-euro-nmd.eu/innovative-and-sustainable-fair-solution-for-ern-euro-nmd/.
- PA Harris, R Taylor, R Thielke, J Payne, N Gonzalez, JG. Conde, Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377-81.
